Share
Introduction
In industrial environments, there are numerous environmental factors which can cause corrosion of circuit boards in electronic process measurement and control systems. Among these are temperature, humidity, and gaseous pollutants. Of these, gaseous pollutants are the most destructive.
Gaseous pollution today is caused primarily by the burning of fuels in power plants, factories, commercial and domestic buildings, and automobiles. The two main types of gaseous industrial air pollutants can be classified either as acidic or oxidizing. Over the years, these two types have merged and now the three main pollutant gases found throughout the industrialized world are sulfur dioxide (SO2), ozone (O3), and nitrogen dioxide (NO2). Others of primary concern include chlorides (chlorine [Cl2] and hydrogen chloride [HCl]), acetic acid (CH3COOH), and formaldehyde (HCHO).
In 1985, a standard was developed to classify air quality with respect to the effects airborne contaminants could have equipment reliability. Generally, this standard calls for concentrations of gaseous pollutants to be maintained as low as attainable by gas-phase air filtration. The most commonly cited control levels for gaseous pollutants are shown below.
| Hydrogen sulfide (H2S) | <3 ppb |
| Sulfur dioxide, sulfur trioxide (SO2, SO3) | <10 ppb |
| Chlorine (Cl2) | <1 ppb |
| Nitrogen oxides (NOX) | <50 ppb |
| Hydrogen fluoride (HF) | <1 ppb |
| Ammonia (NH3) | <500 ppb |
| Ozone (O3) | <2 ppb |
Air Monitoring Tools and Techniques
Air monitoring is central to any environmental control program for achieving and maintaining air quality standards based on the presence (or absence) of gaseous air pollutants. Such monitoring can also provide the short-term data required to manage and mitigate contaminant-specific episodes. In addition to direct application to contamination control programs, air monitoring data may be employed for (1) the evaluation of long-term air quality trends in a facility and (2) research studies designed to determine relationships, if any, between pollutant levels and possible damaging effects. Air quality measurements in preservation environments often make stringent demands on monitoring instrumentation and methodologies. Special modifications and protocols are often needed to adapt the techniques for use in these environments.
Several characteristics of any measurement technique must be evaluated to determine its appropriateness for use in air quality monitoring. Among the more important characteristics are sensitivity, cost, and complexity. Sensitivity is a particularly demanding parameter for environments where near-ambient levels of many pollutants may be encountered and control levels are approaching the sub-parts per billion (ppb) level. Likewise, cost may be quite important when deciding on a measurement technique, particularly in large surveys. A last point of consideration is the complexity of the technique and the degree of skill and training required to obtain quality results. Other factors deserving consideration are selectivity and portability. Most measurement techniques are not optimized for these parameters, and one must weigh the various characteristics to best meet the desired goals. Often trade-offs will be necessary in selecting the techniques to be used for a specific study.
Reactivity Monitoring
Even though it is possible to identify and quantify (almost) all chemical species one may encounter in preservation environments, the question remains “What do I do with this information?” Because of this, many industries have turned to environmental classification via what is referred to as reactivity, or corrosion, monitoring. The validity for this air monitoring technique lies in the fact that many of the pollutants targeted for control are corrosive in nature and, therefore, can be effectively measured using this technique.
Reactivity monitoring can characterize the destructive potential of an environment. The growth of various corrosion films on specially prepared copper and silver sensors provides an excellent indication of the type(s) and level(s) of essentially all corrosive chemical species present in the local environment. They give a direct cause and effect relationship between specific levels of gaseous pollutants and the damage they may cause to copper and/or silver components of circuit boards. Both passive and real-time reactivity monitors are currently available from Purafil and each can be used to gather essential information on gaseous pollutants and their levels in the environment.
Purafil Corrosion Classification Coupons (CCCs).
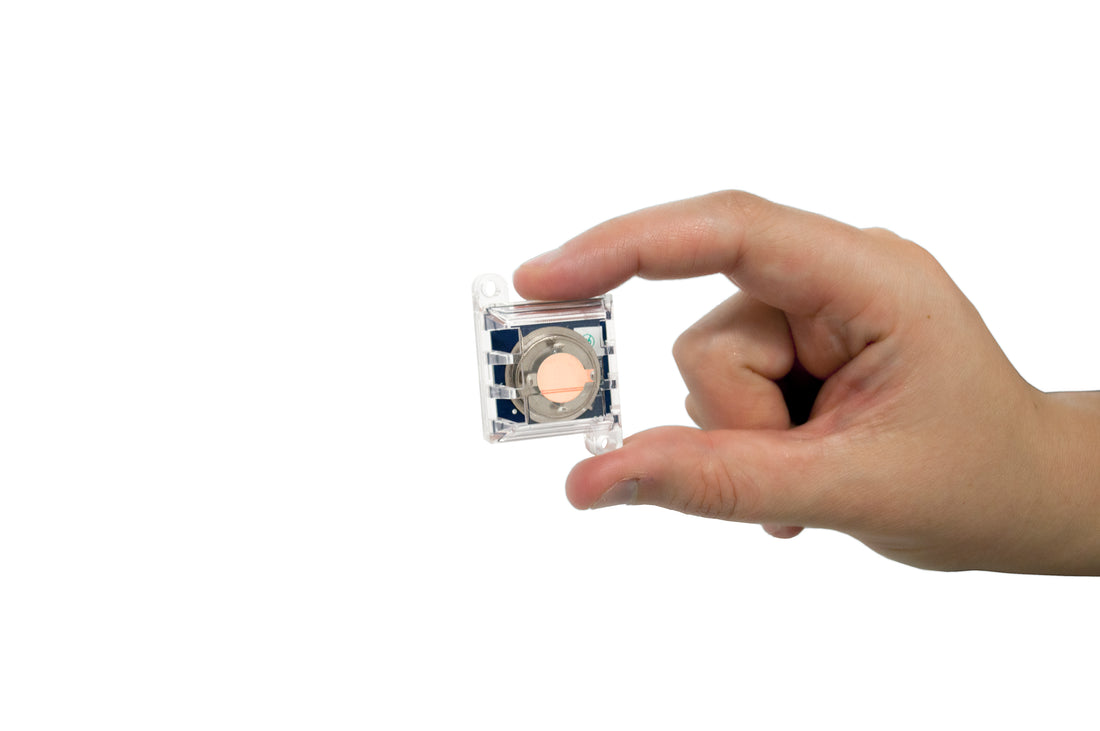
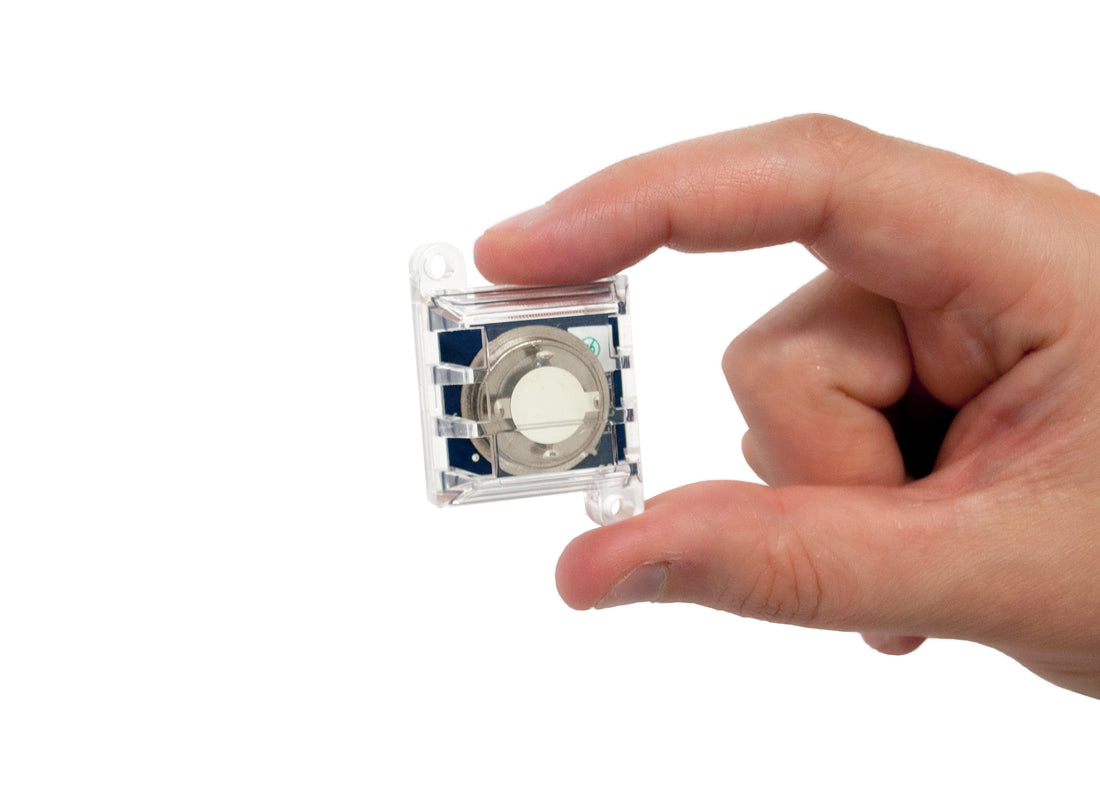
CCCs are passive monitors typically exposed to the environment for a period of 30-90 days and then analyzed for the amount and type of corrosion which has formed (Figure 1). This technique can provide cumulative reactivity rates, an assessment of “average” environmental conditions over time, and an indication of the type(s) and relative level(s) of corrosive gaseous pollutants.

CCCs may be used to indicate the presence of SO2, O3, NO2, Cl2, and many other corrosive materials, which can cause deterioration of electronic devices. CCCs originally used only copper reactivity to establish environmental classifications. However, copper is not sufficiently sensitive to many of those pollutants ubiquitous to many industrial environments. Further, copper coupons cannot detect the presence of chlorine, a particularly dangerous contaminant to metals.
With this in mind, Purafil developed the use of silver reactivity monitoring for these environments. Silver is sensitive to chlorine and, when used with copper reactivity monitoring, can be used detect changes in the levels of gaseous pollutants in the ambient environment as small as 1 ppb and differentiate between different classes of contaminants.
The corrosion reported from reactivity monitoring with CCCs is the sum of individual corrosion films. For copper coupons, sulfide and oxide films are most commonly produced and are reported as copper sulfide (Cu2S) and copper oxide (Cu2O), respectively. For silver coupons, sulfide, chloride, and oxide films may be produced and are reported as silver sulfide (Ag2S), silver chloride (AgCl), and silver oxide (Ag2O), respectively. Each coupon is analyzed as to the type and amount of film present and its relative contribution to the total corrosion produced.1,2
Learn More About Purafil’s CCC
Purafil Electronic Reliability Monitors (ERMs)
One consideration faced in designing an air quality monitoring program is the choice of passive vs. active sampling. The immediate feedback of an active monitor is a most desirable aspect and is what often precludes the use of passive monitors. The main limitation in the use of CCCs is their inability to provide a continuous environmental classification. To address this, reactivity monitoring has been taken a step farther through the development of a real-time monitoring device employing metal-plated quartz crystal microbalances (QCMs).3,4,5. These microprocessor-controlled devices are able to measure the total environmental corrosion attributable to gaseous pollutants. ERMs employing QCMs can detect and record changes <1 ppb. This ability is regarded as one of the main requirements for any real-time monitoring protocol to be used in preservation environments.

To date, there is only one commercially available ERM employing copper and silver-plated QCMs that can provide real-time information on the amount of corrosion forming due to the presence of gaseous pollutants. The OnGuard™ Smart ERM (Figure 2) measures corrosion on a continuous basis which allows for preventive action to be taken before severe damage has occurred. Appropriate reactivity and alarm levels for specific applications can be easily adjusted.
The OnGuard Smart provides live data feed and logs data to help evaluate trends. It can be operated by independently as a battery-operated data logger, wired directly into a central computer system via a 4-20mA connection, or via the internet via ethernet cable or Wi-Fi. Up-to-the-minute information on the levels of corrosive contaminants can be obtained and environmental classification databases can be established and maintained to provide historical data.
Learn More about the OnGuard Smart
Environmental Classifications
Table 1 lists a standard classification scheme, as published in ANSI/ISA standard 71.04-20136, which directly correlates corrosion rates to environmental classifications. These have recently been refined based on the results of testing and the specific needs of this market. Typical uses of reactivity monitoring to date have been for the characterization of outdoor air used for ventilation, the identification of “hot spots” within a facility, and the effectiveness of various preventive measures. This table also shows us the correlation between the corrosion levels and the maximum gas concentrations allowable within that particular corrosion class.

A G1 environment is defined as: An environment sufficiently well-controlled such that corrosion is not a factor in determining equipment reliability.
BOTH the copper and silver corrosion rates should be class G1 or better unless otherwise agreed upon. The individual corrosion films quantified using reactivity monitoring may be used to further characterize the environment and to determine the proper control strategies. Based upon these recommended control levels and test results from laboratory and field-exposed silver coupons, acceptance criteria relevant to these applications has been determined. These criteria consider total corrosion as well as the relative contribution of each individual corrosion film. The control specifications for the individual corrosion films are listed in TABLE 2. These specifications are more general in their application than those listed above and are most often used for the characterization of an environment prior to the implementation of pollutant control measures.

If the total corrosion AND each individual corrosion film meets the recommended criteria, the local environment in which that specific coupon has been exposed meets the requirements of a Class G1 classification. ANY of the criteria which are not met indicates that the local environment may not be sufficiently well-controlled to minimize the corrosion of sensitive electronic equipment due to the presence of gaseous pollutants. Steps should be taken to determine what problems exist and what corrective actions may be appropriate.
CCC Results and Discussion
When interpreting the analysis results for the individual corrosion films, the detection of a silver sulfide (Ag2S) film without a corresponding copper sulfide (Cu2S) film usually indicates the presence of oxidized forms of sulfur such as sulfur dioxide (SO2) and sulfur trioxide (SO3).
- Oxidized forms of sulfur are generated as combustion products of sulfur-bearing fossil fuels. Low parts per billion levels of sulfur oxides can passivate reactive metals and thus retard corrosion. At higher levels they attack certain types of masonry, metals, elastomers, and plastics. The reaction with masonry and metals normally occurs when these gases dissolve in water to form sulfurous and sulfuric acid.
- Iron is the chief metal to suffer from the presence of sulfur dioxide. Iron corrodes to rust electrolytically. This means that both moisture and an electrolyte must be present on the iron surface. All water-soluble salts, acid, and alkalis form electrolytes. Those electrolytes that attract moisture, form soluble corrosion products, and are non-volatile, are the most corrosive. Sulfuric acid and the ammonium sulfate to which it is often partly converted fulfill all these conditions.
- When both films are detected it most often indicates of the presence of active sulfur compounds such as elemental sulfur, hydrogen sulfide (H2S), and organic sulfur compounds (e.g., mercaptans) as well. When both films are present and the amount of Cu2S is greater than 50% of the total corrosion, this is further evidence of the presence of active sulfur compounds in the subject environment.
- Active sulfur compounds include hydrogen sulfide, elemental sulfur, and organic sulfur compounds such as the mercaptans. When present at low parts per billion levels, they rapidly attack copper, silver, aluminum, and iron alloys. The presence of moisture and small amounts of inorganic chlorine compounds greatly accelerates sulfide corrosion. Note, however, that attack still occurs in low relative humidity environments. Active sulfurs rank with inorganic chlorides as the predominant cause of atmospheric corrosion.
- Chloride corrosion (AgCl) indicates the presence of (an) inorganic chlorine compound(s), e.g., chlorine (Cl2), chlorine dioxide (ClO2), hydrogen chloride (HCl). Elevated levels of chloride (halogen) contamination can also serve to effectively mask any evidence of sulfur contamination on the corresponding copper coupons and can cause a large “unknown” copper corrosion film to appear.
- Chlorine contamination, whether as chlorine or hydrogen chloride, are a most dangerous contaminant for metals. At elevated levels, many elastomers and some plastics are oxidized by exposure to chlorinated gases.
- Specific care must be given to materials that are exposed to environments containing chlorinated contaminants.
Conclusions
The amount of corrosion forming over any given period is a primary indicator of how well-controlled an environment may be. Where gas filtration is employed to maintain the interior concentrations of gaseous pollutants as low as possible, reactivity levels well within the general and specific acceptance criteria can be easily attained. It is felt that if an environment exhibits reactivity rate of G1 (<300 of copper corrosion /30 days AND <200 of silver corrosion /30 days), there is little else that can be done, economically, to improve the environment.
When the total amount of copper or silver corrosion measured is above an ISA G1 severity level and the presence of sulfur oxides, chlorides and/or active sulfur has been confirmed, the air should be treated to remove these contaminants and to prevent corrosion. A Purafil chemical filtration system should be able to specifically address the sulfur and chlorine contamination.
In general, chemical filtration should be installed at the makeup intakes to reduce and maintain chemical contamination at acceptable levels. The detection of active sulfur and/or chlorine contamination is particularly damaging to metals – even at very low levels and steps should be taken to reduce contaminant levels to those which would not have effects on metal components.
It is further recommended that a reactivity monitoring program be established – either with CCCs or the OnGuard Smart Monitor – to provide a continuous assessment air quality. While information on individual contaminant species can be obtained using the CCCs, real-time reactivity monitoring with the OnGuard Smart can provide a more accurate assessment of the total corrosion being formed due to the presence of chemical contaminants. Reactivity monitoring can also be used to measure the performance of Purafil, Inc. chemical filter systems (if installed) and serve as a guide for media replacement.
Direct gas monitoring may be indicated to determine the source(s) of sulfur and/or chlorine corrosion reported. This could help determine if these results were typical of this location, an anomaly, due to episodic events, or if there are some other gaseous contaminants present which may have synergistic effects and need to be accounted for.
- Abbott, W.H., “The Effects of Operating Environments on Electrical and Electronic Equipment Reliability in the Pulp and Paper Industry,” IEEE Conference Record, Institute of Electrical and Electronic Engineers, Inc., New York, 1983.
- Rice, D.W., et al., “Atmospheric Corrosion of Copper and Silver,” Electrochemical Society, 128(2), pp 275-284, 1981.
- W.G. England, et al., “Applications of a Real-Time Electronic Contact Corrosion Monitor,” Proceedings of Advances in Instrumentation and Control, Vol 46: pp 929-955, Instrument Society of America, Anaheim, 1991.
- A.J. Weiller, “Electronic Monitoring of Indoor Atmospheric Pollutants,” Proceedings of Healthy Buildings ’94, pp 241-243, National Coalition on Indoor Air Quality, 1994.
- Forslund, M., “A Quartz Crystal Microbalance Probe for in situ Atmospheric Corrosion Monitoring,” Department of Materials Science and Engineering, Division of Corrosion Science, Royal Institute of Technology, pp. 1-44, Stockholm, 1996.
- ISA Standard ANSI/ISA-S71.04-2013, “Environmental Conditions for Process Measurement and Control Systems: Airborne Contaminants,” International Society for Measurement and Control, Research Triangle Park, NC, 2013.










0 comment